Publications
Selected publications are below. A complete list can be found here

Damilare Olorunnisola, Chidinma G. Olorunnisola, Ephraim Akor, Moses O. Alfred, Nathaniel B. Bolujoko, Christina Günter, Costas Michael, Andreas Taubert, Harshadrai M. Rawel, Timothy L. Easun, Chukwunonso P. Okoli, Despo Fatta-Kassinos, Emmanuel I. Unuabonah
Microchemical Journal, 2024, 200, 110355
DOI: 10.1016/j.microc.2024.110355
How could you remove contaminants of emerging concern from water?
Solid phase extraction is an established technique for concentrating or removing organic contaminants from aqueous solutions, but is costly and often hard to recycle, making it materially wasteful. In this Nigerian-led collaboration, we explored the use of cellulose, a cheap and readily available material, modified by crosslinking, as a new low-cost solid phase extraction material that can be readily regenerated. The new material showed both excellent adsorption of two Contaminants of Emerging Concern (CPA and BPA) as well as very good limits of detection for both in the analysis of trace contamination of water.
Daniel J. Cerasale, Dominic C. Ward & Timothy L. Easun
Nature Reviews Chemistry, 2022, 6, 930
DOI: 10.1038/s41570-021-00336-8
What do MOFs and their guests do over time? How do we know?
Understanding the time-dependent behaviours of MOFs and their guests is critical to understanding and developing these fascinating materials into the future. This Review provides an overview of current research into the temporal evolution of MOF structures and properties by analysing the time-resolved experimental techniques that can be used to monitor such behaviours. We focus on innovative techniques, while also discussing older methods often used in other chemical systems. Four areas are examined: MOF formation, guest motion, electron motion and framework motion. In each area, we highlight the disparity between the relatively small amount of (published) research on key time-dependent phenomena and the enormous scope for acquiring the wider and deeper understanding that is essential for the future of the field.

Enabling batch and microfluidic non-thermal plasma chemistry: reactor design and testing
P. Roszkowska, A. Dickenson, J. E. Higham, T. L. Easun, J. L. Walsh &
A. G. Slater
Lab Chip, 2023, 23, 2720
DOI: 10.1039/D3LC00016H
Can we do chemistry using non-thermal plasmas?
In a departure from MOF chemistry, we used an interdisciplinary approach that incorporated Tim's spectroscopic expertise to study plasma chemistry in batch and microfluidic flow conditions. Non-thermal plasma (NTP) is a promising state of matter for carrying out chemical reactions. NTP offers high densities of reactive species, without the need for a catalyst, while operating at atmospheric pressure and remaining at moderate temperature. Despite its potential, NTP cannot be used comprehensively in reactions until the complex interactions of NTP and liquids are better understood. To achieve this, NTP reactors that can overcome challenges with solvent evaporation, enable inline data collection, and achieve high selectivity, high yield, and high throughput are required. Here, we detail the construction of i) a microfluidic reactor for chemical reactions using NTP in organic solvents and ii) a corresponding batch setup for control studies and scale-up. The use of microfluidics enables controlled generation of NTP and subsequent mixing with reaction media without loss of solvent. The construction of a low-cost custom mount enables inline optical emission spectroscopy using a fibre optic probe at points along the fluidic pathway, which is used to probe species arising from NTP interacting with solvents. We demonstrate the decomposition of methylene blue in both reactors, developing an underpinning framework for applications in NTP chemical synthesis.
Magdalene W. S. Chong, Stephen P. Argent, Florian Moreau, William J. F. Trenholme, Christopher G. Morris, William Lewis, Timothy L. Easun, Martin Schröder
Chem. Eur. J., 2022, 28, 52, e202201188
DOI: 10.1002/chem.202201188
Can you make a flexible MOF using copper and a short linker?
A 2D coordination network was synthesised from commercial reagents and displays two distinct coordination environments of copper to carboxylate and salicylaldehydato moieties of the ligand. Solvent exchange of the synthesized network shows retention of framework connectivity coupled with unanticipated framework flexibility to accommodate tetrahydrofuran. The activated framework selectively adsorbs carbon dioxide and C2 hydrocarbons over methane.



Selective Gas Uptake and Rotational Dynamics in a (3,24)-Connected Metal–Organic Framework Material
William J. F. Trenholme, Daniil I. Kolokolov, Michelle Bound, Stephen P. Argent, Jamie A. Gould, Jiangnan Li, Sarah A. Barnett, Alexander J. Blake, Alexander G. Stepanov, Elena Besley, Timothy L. Easun*, Sihai Yang, and Martin Schröder
J. Am. Chem. Soc. 2021, 143, 9, 3348
DOI: 10.1021/jacs.0c11202
How do rings spin in a MOF and how are the guests involved?
The desolvated (3,24)-connected Cu-based metal–organic framework MFM-160a exhibits excellent high-pressure uptake of carbon dioxide and highly selective separation of C2 hydrocarbons from methane at 1 bar pressure. Solid-state 2H NMR spectroscopic studies on the partially deuterated analogue confirm an ultra-low barrier (∼2 kJ/mol) to rotation of the phenyl group in the activated MOF and a rotation rate 5 orders of magnitude slower than usually observed for solid-state materials. Upon introduction of carbon dioxide or ethene into desolvated MFM-160a, this rate of rotation was found to increase with increasing gas pressure, a phenomenon attributed to the weakening of an intramolecular hydrogen bond in the triazine-containing linker upon gas binding. DFT calculations of binding energies and interactions of CO2 and C2H2 around the triazine core are entirely consistent with the 2H NMR spectroscopic observations, and putting all the sources of information together provides a detailed picture of how ring rotation and gust sorption interact in this MOF material.
Exploiting in situ NMR to monitor the formation of a metal–organic framework
Corey L. Jones, Colan E. Hughes, Hamish H.-M. Yeung, Alison Paul, Kenneth D.M. Harris, Timothy L. Easun
Chemical Science, 2021, 12, 4, 1486
DOI: 10.1039/D0SC04892E
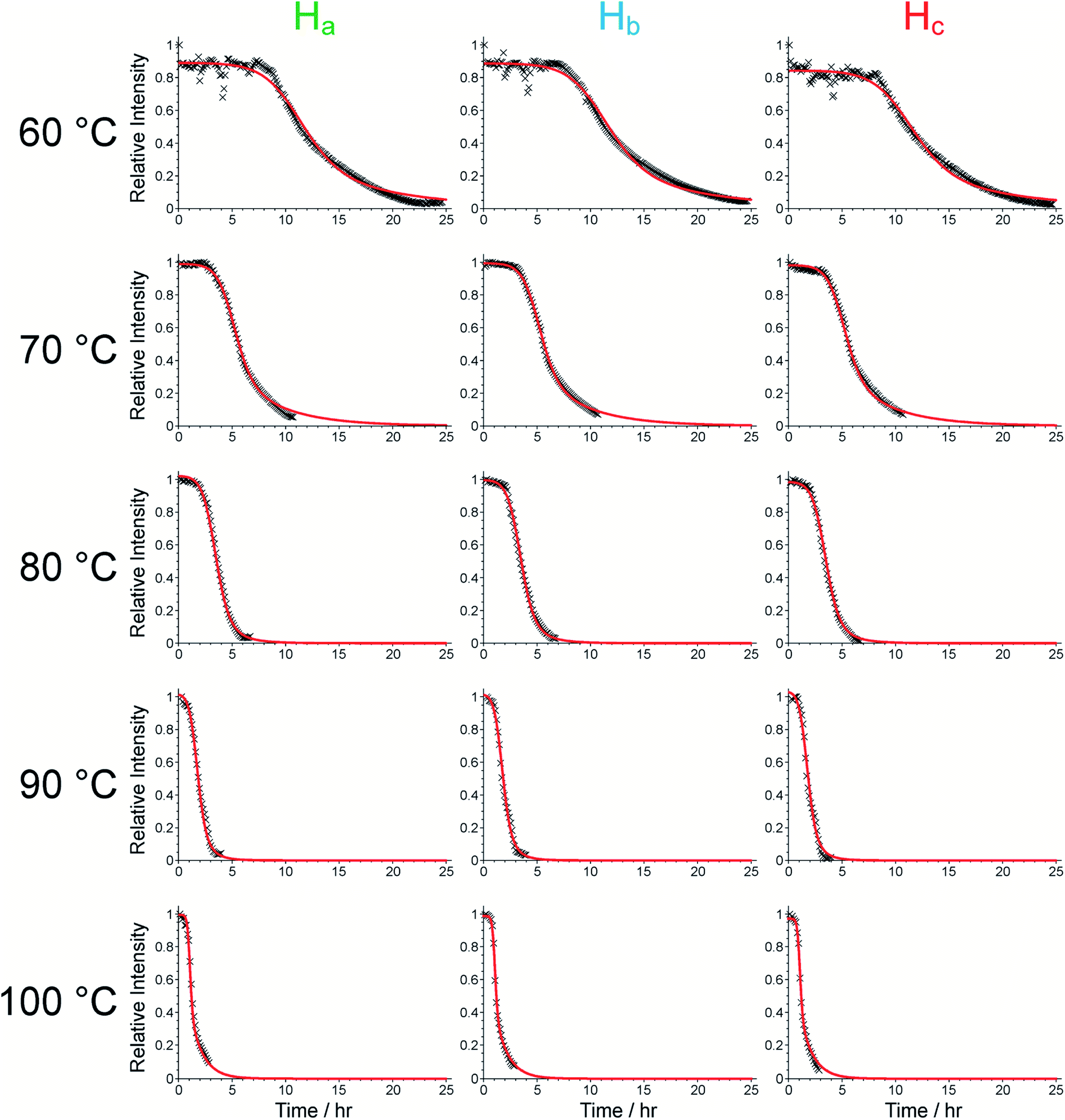
How do MOFs form?
The formation processes of metal–organic frameworks are becoming more widely researched using in situ techniques, although there remains a scarcity of NMR studies in this field. In this work, the synthesis of framework MFM-500(Ni) has been investigated using an in situ NMR strategy that provides information on the time-evolution of the reaction and crystallization process. In our in situ NMR study of MFM-500(Ni) formation, liquid-phase 1H NMR data recorded as a function of time at fixed temperatures (between 60 and 100 °C) afford qualitative information on the solution-phase processes and quantitative information on the kinetics of crystallization, allowing the activation energies for nucleation (61.4 ± 9.7 kJ/mol) and growth (72.9 ± 8.6 kJ/mol) to be determined. Ex situ small-angle X-ray scattering studies (at 80 °C) provide complementary nanoscale information on the rapid self-assembly prior to MOF crystallization and in situ powder X-ray diffraction confirms that the only crystalline phase present during the reaction (at 90 °C) is phase-pure MFM-500(Ni). This work demonstrates that in situ NMR experiments can shed new light on MOF synthesis, opening up the technique to provide better understanding of how MOFs are formed.

Aisha Asghar, Naseem Iqbal, Tayyaba Noor, Benson M. Kariuki, Luke Kidwell, Timothy L. Easun
Green Chemistry, 2021, 23, 3, 1220
DOI: 10.1039/D0GC03292A
Can you make a MOF using electrochemistry?
The use of electrochemical synthesis of MOFs is still very limited in the literature. In this study Mn–DABDC (DABDC = diaminobenzenedicarboxylate, or 2,5-diaminoterephthalate) MOF was synthesised both via an electrochemical method, to make Mn–DABDC(ES), and via a conventional solvothermal approach, to make Mn–DABDC(ST). A Mn–BDC (BDC = benzenedicarboxylate) MOF was also prepared by a conventional solvothermal method for gas uptake capacity comparison. Investigation of the electrochemical synthesis parameters demonstrated that current density, electrolyte amount and reaction time were the most significant factors affecting crystal synthesis and product yield. The best conditions found for obtaining a crystalline MOF with high yield (93%) were identified, allowing for a relatively fast MOF synthesis, important for reducing synthesis cost compared with conventional hydrothermal and solvothermal methods. The electrochemically synthesized MOF has high carbon dioxide uptake (92.4 wt% at 15 bar and 273 K) and hydrogen uptake (12.3 wt% at 80 bar and 77 K). This is the first amine-based manganese MOF synthesized electrochemically, and the method has excellent potential for reducing large-scale MOF production costs.
Aisha Asghar, Naseem Iqbal, Tayyaba Noor, Majid Ali, Timothy L. Easun
Nanomaterials, 2019, 9(8), 1063
DOI: 10.3390/nano9081063
How can carbon dioxide uptake in a MOF be improved?
Herein we report a facile, efficient, low cost, and easily scalable route for an amine-functionalized MOF (metal organic framework) synthesis. Cu-BDC⊃ HMTA (HMTA= hexamethylenetetramine) has high nitrogen content and improved thermal stability when compared with the previously reported and well-studied parent Cu-BDC MOF (BDC= 1, 4-benzenedicarboxylate). Cu-BDC⊃ HMTA was obtained via the same synthetic method, but with the addition of HMTA in a single step synthesis. Thermogravimetric studies reveal that Cu-BDC⊃ HMTA is more thermally stable than Cu-BDC MOF. Cu-BDC⊃ HMTA exhibited a CO 2 uptake of 21.2 wt% at 273 K and 1 bar, which compares favorably to other nitrogen-containing MOF materials.


Tamoghna Mitra, Florian Moreau, Adam Nevin, Carlo U. Perotto, Alex Summerfield, E. Stephen Davies, Elizabeth A. Gibson, Timothy L. Easun, Martin Schröder
Chemical Science, 2018, 9, 31, 6572
DOI: 10.1039/C8SC00803E
How can you study electrochemistry in a MOF?
The application of metal–organic framework (MOF) materials in electrochemical and electrochromic devices remains rare. One of the main reasons for this is the inability to readily access their detailed electrochemistry. The inherent insolubility of these materials does not allow interrogation by traditional solution-based electrochemical or spectroscopic methods. In this study, we report a straightforward alternative approach to the spectroelectrochemical study of MOFs. We have used two systems as exemplars in this study, MFM-186 and MFM-180. The method involves chemical modification of a working electrode to attach MOF materials without using corrosive reagents such as inorganic acids or bases which otherwise could limit their application in device development. MFM-186 demonstrates the formation of a stable radical species [MFM-186]˙+ on electrochemical oxidation, and this has been characterised by electrochemical, spectroelectrochemical and EPR spectroscopic techniques coupled to DFT analysis.
Corey L Jones, Elizabeth A Marsden, Adam C Nevin, Benson M Kariuki, Mohan M Bhadbhade, Adam D Martin, Timothy L Easun
Royal Society Open Science, 2017, 4, 12, 171064
DOI: 10.1098/rsos.171064
What directs the geometry of a linker of limited flexibility in the synthesis of coordination polymers?
A series of new group 2 coordination polymers, MgL ={MgL(H2O)(DMF)0.75}∞, CaL = {CaL(DMF)2}∞, SrL = {SrL(H2O)0.5}∞ and BaL = {BaL(H2O)0.5}∞, were synthesized using a flexible benzimidazolone diacetic acid linker (H2L) in which the two carboxylic acid binding sites are connected to a planar core via {–CH2–} spacers that can freely rotate in solution. In a ‘curiosity-led' diversion from group 2 metals, the first row transition metal salts Mn2+, Cu2+ and Zn2+ were also reacted with L to yield crystals of MnL = {MnL(DMF)(H2O)3.33}∞, Cu3L2 = {Cu3L2(DMF)2(CHO2)2}∞ and ZnL = {ZnL(DMF)}∞. Crystal structures were obtained for all seven materials. All structures form as two-dimensional sheets and contain six-coordinate centres, with the exception of ZnL, which displays tetrahedrally coordinated metal centres, and Cu3L2, which contains square planar coordinated metal centres and Cu paddle-wheels. In each structure, the linker adopts one of two distinct conformations, with the carboxylate groups either cis or trans with respect to the planar core. All materials were also characterized by powder X-ray diffraction and thermogravimetric analysis.


MOF the beaten track: unusual structures and uncommon applications of metal–organic frameworks
Alexander J. Tansell, Corey L. Jones and Timothy L. Easun
Chemistry Central Journal, 2017, 11:100, 1-16
DOI: 10.1186/s13065-017-0330-0
Ever wondered what else MOFs can do?
Over the past few decades, metal–organic frameworks (MOFs) have proved themselves as strong contenders in the world of porous materials, standing alongside established classes of compounds such as zeolites and activated carbons. Following extensive investigation into the porosity of these materials and their gas uptake properties, the MOF community are now branching away from these heavily researched areas, and venturing into unexplored avenues. Ranging from novel synthetic routes to post-synthetic functionalisation of frameworks, host–guest properties to sensing abilities, this review takes a sidestep away from increasingly ‘traditional’ approaches in the field, and details some of the more curious qualities of this relatively young family of materials.
Florian Moreau, Ivan da Silva, Nada H. Al Smail, Timothy L. Easun, Mathew Savage, Harry G. W. Godfrey, Stewart F. Parker, Pascal Manuel, Sihai Yang, and Martin Schröder
Nature Communications, 2017, 8, 14085
How do you get a lot of acetylene in a MOF?
Understanding the mechanism of gas-sorbent interactions is of fundamental importance for the design of improved gas storage materials. Here we report the binding domains of carbon dioxide and acetylene in a tetra-amide functionalized metal-organic framework, MFM-188, at crystallographic resolution. Although exhibiting moderate porosity, desolvated MFM-188a exhibits exceptionally high carbon dioxide and acetylene adsorption uptakes with the latter (232 cm3 g−1 at 295 K and 1 bar) being the highest value observed for porous solids under these conditions to the best of our knowledge. Neutron diffraction and inelastic neutron scattering studies enable the direct observation of the role of amide groups in substrate binding, representing an example of probing gas-amide binding interactions by such experiments. This study reveals that the combination of polyamide groups, open metal sites, appropriate pore geometry and cooperative binding between guest molecules is responsible for the high uptakes of acetylene and carbon dioxide in MFM-188a.

